Regarding experiments with genetically modified organisms
Genetically modified organism diffusion prevention measures (P1A, P2A level) are in effect within the Animal Facility.Due to this, the researchers are able to produce and breed genetically modified animals in the facility.
Procedures for starting experiments
If you wish to create/care for genetically modified animals within the Animal Facility, refer to the following page, join the education and training course held by the Kumamoto University genetically modified organism Type 2 Usage Safety Commission and complete the necessary preliminary procedures.[Reference Page]
- Kumamoto University genetic experiment facility - information about gene recombination experiments
- Ministry of Education Life Sciences Plaza - gene recombination experiments
- Gene recombination experiment application relating to genetically modified mice (Misao Suzuki, 2007 Pharmacological Daily Magazine #129, P. 320 - 324)
In order to perform gene recombination experiments, researchers will need to comply with and understand the following laws.
Laws regarding gene recombination experiments
International agreements
In performing experiments using genetically modified organisms, they must comply with the Cartagena Protocol international treaty.The Cartagena Protocol is a treaty to preserve biodiversity on earth, and prescribes measures to prevent living (genetically) modified organisms (LMO's) created by modern biotechnology from adversely affecting biodiversity.
Nearly every country, with the exception of the United States, has ratified and signed the Cartagena Protocol.
Domestic law
In Japan, we must follow the following laws to protect biodiversity.These laws specify detailed measures for preventing the diffusion of genetically modified organisms (diffusion prevention measures) caused from experimenting outside of laboratories.
- Acts on biodiversity based on regulations for the use of genetically modified organisms, etc.
- Ordinance to define diffusion prevention measures to be taken in regards to research and development, etc., of type 2 living modified organisms.
- Case to determine a certified host-vector system or similar based on provisions of Ordinance to define diffusion prevention measures to be taken in regards to research and development, etc., of type 2 living modified organisms.
Gene recombination experiment classification
In light of the above domestic laws, the following experiments using genetically modified animals are classified as "experiments using animals.""Experiments using animals" are further divided into animal creation experiments and animal inoculation experiments.
Creation and use of genetically modified animals (Tg mice, etc.) are considered animal creation experiments, as are experiments dealing with simple breeding of genetically modified mice.
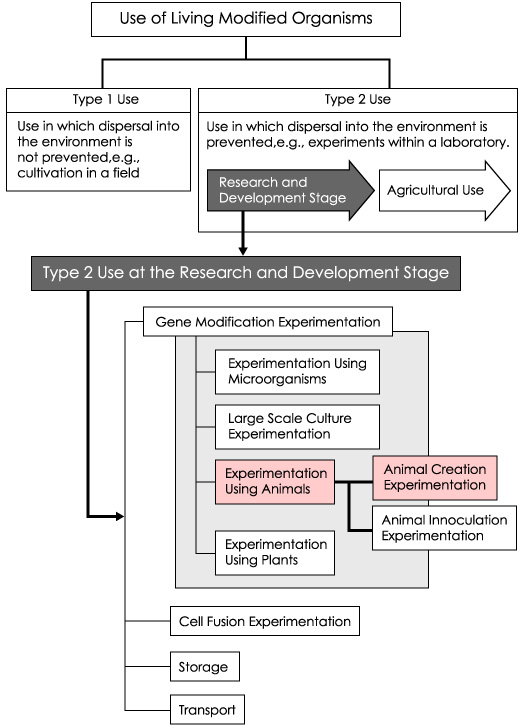
Diffusion prevention measures
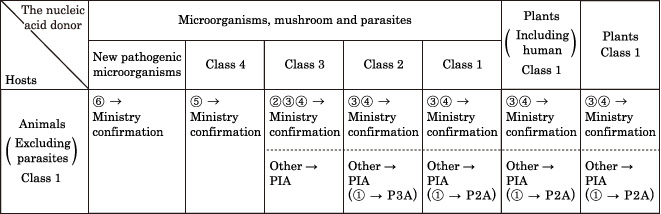
Diffusion prevention measures to be taken for animal creation experiments depend on genetically modified animal hosts and nucleic acid donors.The nucleic acid donor is classified by its virulence and transmissibility, and this determines the diffusion prevention measures to be taken. The host of the animal experiment is referred to as the "animal," and "animals" are classified as Class 1.
| 1. Class 1 | Of microorganisms, mushroom and parasites, those which are not pathogenic to animals belonging to Mammalia and Aves (including Homo. Hereinafter “mammals”) and are stipulated by the Minister of Education, Culture, Sports, Science and Technology, and animals (including Home and excluding parasites) and plants |
|---|---|
| 2. Class 2 | Of microorganisms, mushroom and parasites, those which are low in pathogenicity to mammals and are stipulated the Minister of Education, Culture, Sports, Science and Technology |
| 3. Class 3 | Of microorganisms and mushroom, those which are high in pathogenicity to mammals and low in propagation and are stipulated by the Minister of Education, Culture, Sports, Science and Technology |
| IV. Class 4 | Of microorganisms, those which are high in pathogenicity to mammals and high in propagation and are stipulated by the Minister of Education, Culture, Sports, Science and Technology |
Article 3: The names of experiment classification and recipient organisms or donor organisms which belong to the experiment classifications shall be as stipulated respectively in the left columns and the right columns of the following table.
Referring to the following table, determine the diffusion prevention measures (P1A - P3A) that should be taken.
P1A level measures are fine for most experiments using animals as hosts, however, if the nucleic acid donor matches with (1) to (6) below, Ministry confirmation and P2A or P3A measures are necessary.
("Ministry confirmation" means that the Ministry of Culture, Sports, Science and Technology instead of the University committee must be consulted for deciding which prevention measures should be taken.)
1. If donated nucleic acids are anticipated to greatly enhance pathogenicity towards mammals: P2A for nucleic acid donors with Class 1 experimental classification, or P3A for Class 2.
2. Among living modified organisms not making use of an authorized host-vector system for which the nucleic acid donor experimental classification is Class 3, those that enhance host pathogencity and for which donated nucleic acids are either unidentified or identified.
3. Nucleic acids that include genes related to protein toxins with a 50% lethal dose for mammals of 100μg or less per 1kg of body weight.
4. Nucleic acids that include genes that give infection receptors of pathogenetic microorganisms (not present in the host) to the host.
5. Use of Class 4 nucleic acid donor experimental classification.
6. Use of new pathogenic microorganisms (those not on the experimental classification list of the R&D Type 2 Act) for nucleic acid donors.
The diffusion prevention measures taken at Kumamoto University's Animal Facility are P1A or P2A level and thus most experiments using genetically modified animals can be performed at the facility.
References:Gene recombination experiment application relating to genetically modified mice (Misao Suzuki, 2007 Pharmacological Daily Magazine #129, P.320 - 324)
If you have questions about gene recombination experiments, please contact the gene experiment facility.
Breeding area in which the diffusion prevention measures are taken
P1A Level
Main Bldg.: All animal care rooms except on the 4th floor -> Floor plan (Internal University use only)New Bldg.: All animal care rooms -> Floor plan (Internal University use only)
P2A Level
Main Bldg. 4th floor animal care rooms -> Floor plan (Internal University use only)NEWS
- New web site is opened.
2016.03.31
SUPPORT
ACTIVITIES
STAFF
Kumamoto University
Institute of Resource Development and Analysis
Center for Animal Resources and Development
〒860-0811
2-2-1 Honjo, Chuo-ku, Kumamoto-shi
TEL :096-373-6550
FAX :096-373-6552
Email :mimura(a)kumamoto-u.ac.jp
Liaison:Imura
Contact List (Internal University use only)
Institute of Resource Development and Analysis
Center for Animal Resources and Development
〒860-0811
2-2-1 Honjo, Chuo-ku, Kumamoto-shi
TEL :096-373-6550
FAX :096-373-6552
Email :mimura(a)kumamoto-u.ac.jp
Liaison:Imura
Contact List (Internal University use only)


















