In Vitro Fertilization using Cryopreserved Spermatozoa
Materials and Equipment
- Cryopreserved spermatozoa in straws
- Female mice superovulated with PMSG and hCG
- FERTIUP® (Preincubation medium: PM; COSMO BIO)
- CARD MEDIUM (COSMO BIO)
- mHTF
- Pipette tips (QSP pipette tip Cat.No.114 Thermo scientific)
- Plastic dishes (35mm x 10mm Cat.No.430588; CORNING)
- Straw connector
- Water bath maintained at 37℃
- Float for thawing
- Micropipettes
- Humidified incubator (37℃, 5% CO2, 95% air)
Procedure
Preparation of the Float for Thawing
- Using some styrofoam and a 50 mL plastic centrifuge tube, make the float as shown in the diagram below.
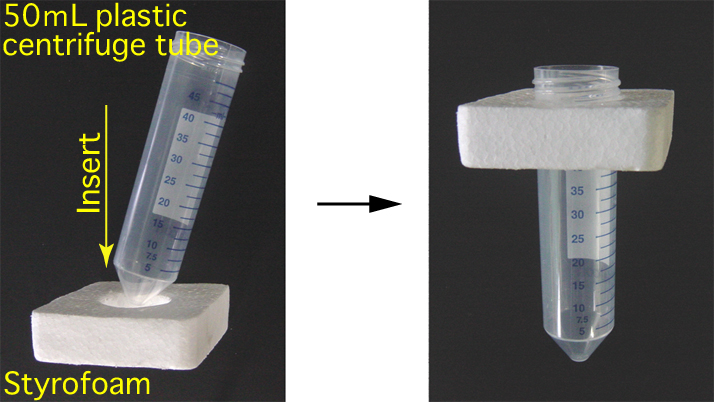
Preparation for Thawing
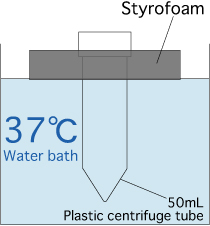
- Prepare a water bath maintained at 37℃.
- Pour water (37℃) into the 50mL plastic centrifuge tube part of the styrofoam/centrifuge tube assembly, and float it in a water bath.
-
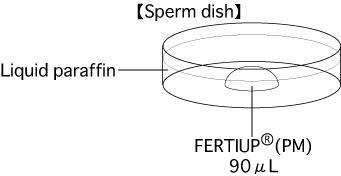
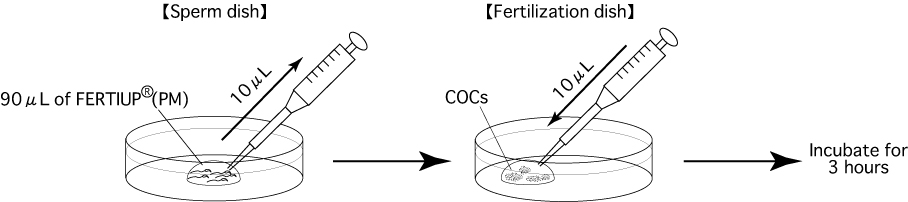
Put 1 drop (90μL / drop) of FERTIUP®(PM) into a dish and cover it with liquid paraffin 30 minutes before thawing a frozen straw, and place the dish in an incubator (37℃, 5% CO2 in air).
-
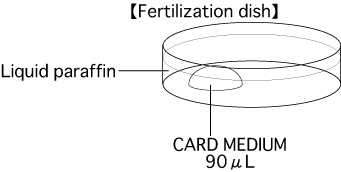
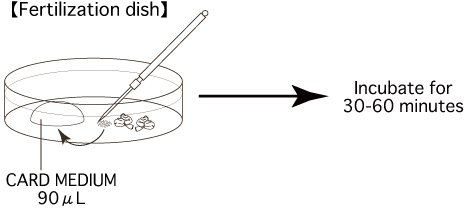
Put 1 drop (90μL / drop) of CARD MEDIUM into a dish and cover it with liquid paraffin 10 minutes before collecting of oocytes, and place the dish in an incubator (37℃, 5% CO2 in air).
Note: There are three different methods of preparing CARD MEDIUM, depending on whether in vitro fertilization will be carried out using fresh, frozen-thawed or cold-temperature transported spermatozoa.
Please refer to the CARD MEDIUM instruction manual.
-
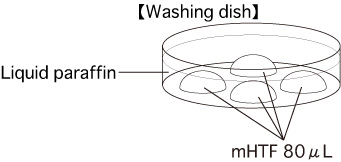
Put 4 drops (80μL / drop) of mHTF into a dish and cover them with liquid paraffin. Place the dish in an incubator (37℃, 5% CO2 in air) for at least 30 minutes.
Collection of Oocytes
- Sacrifice female mice 15-17 hours after an hCG injection and remove the oviducts.
- Using fine, sharp needles, release up to 4-6 cumulus-oocytes-complexes (COCs) masses into each drop of CARD MEDIUM (90μL) (Fertilization dish).
Note: Be sure to carry out all operations, from sacrificing the female and removing her oviducts to introducing the COCs into a drop of CARD MEDIUM, in the shortest time possible (within 30 seconds). Moreover, when carrying out this process alone, do not sacrifice multiple mice at once; instead, sacrifice one mouse and swiftly remove its oviducts before moving on to the next mouse.
- Keep the fertilization dish including COCs in an incubator (37℃, 5% CO2 in air) for 30-60 minutes before insemination.
Thawing the Mouse Spermatozoa
- Remove a frozen straw from the liquid nitrogen and hold it in the air for 5 seconds.
- After completing step 1, immediately immerse the frozen straw in the styrofoam/centrifuge tube assembly (in a water bath maintained at 37℃) for 10 minutes.
Note: To ensure warming of the frozen sperm, completely immerse the part of the straw containing the sperm in the water bath. Furthermore, frozen-thawed mouse spermatozoa are sensitive to environmental changes. If the straw is not kept in the water bath long enough (10 minutes), the motility of the cryopreserved spermatozoa will be reduced. - 10 minutes after immersion, remove the straw from the styrofoam/centrifuge tube assembly.
- Use fine tissues to wipe any water from the straw.
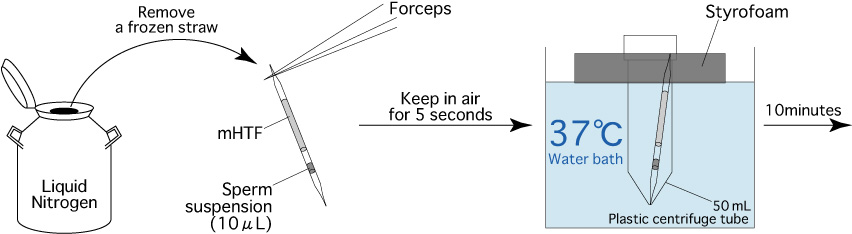
[Thawing of Mouse Spermatozoa]
Transferring and Preincubating the Thawed Sperm Suspension
- Pull the plunger out of the syringe in the straw connector, and cut the straw between the mHTF and the seal.
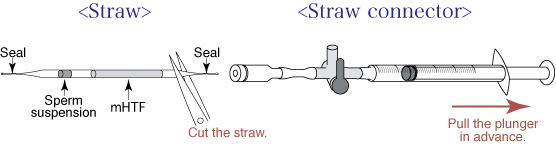
- Insert the cut straw into the straw connector.
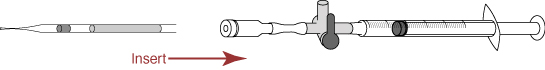
- Because the insertion of the straw into the straw connector creates pressure inside the straw, turn the stopcock in order to release the pressure.
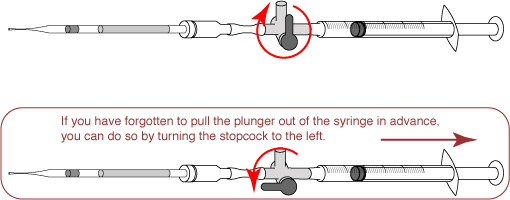
- Return the stopcock to the upwards position, and cut the straw between the seal and the sperm suspension.
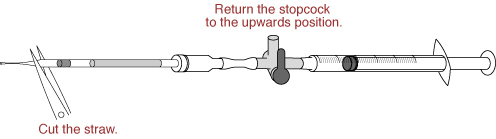
- Push the plunger to transfer only the sperm suspension into the drop of FERTIUP®(PM) (sperm dish), and place the dish in an incubator (37℃, 5% CO2 in air) for 30 minutes.
Note: Do not disturb the dishes containing cryopreserved spermatozoa until they are moving sufficiently within the medium. If the dishes are disturbed before the spermatozoa start to move, then they will not recover full motility.
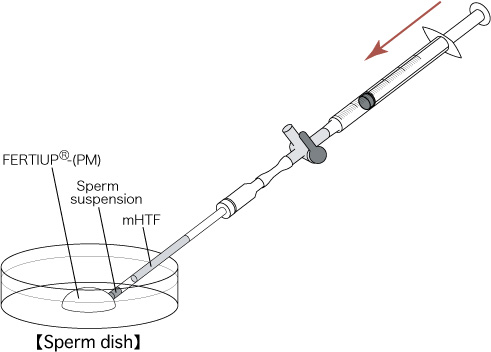
[Transfer of the Thawed Sperm Suspension]
Insemination
- Using a wedge-shaped pipette tip (QSP pipette tip Cat.No.114 Thermo scientific), aspirate 10μL of the preincubated sperm suspension from the edge of the drop.
Comment: Spermatozoa with high motility have a tendency to gather near the edge of the drop. Comment: It is possible to aspirate 10μL of sperm suspension 3-4 times per drop. - Add 10μL of sperm to each drop of fertilizing CARD MEDIUM containing the COCs.
Note: Perform the pipette operation mentioned in steps 1 and 2 as gently as possible. - Incubate the oocytes and spermatozoa for 3 hours in an incubator (37℃, 5% CO2 in air).
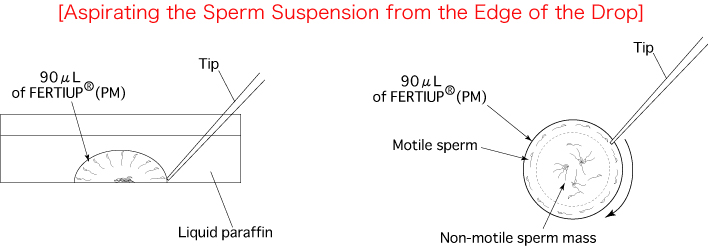
[Aspiration of Preincubated Sperm Suspension and Insemination of Oocytes]
- After incubating for 3 hours, wash the oocytes 3 times in fresh mHTF (80μL) in a washing dish, avoiding transfer of CARD MEDIUM.
Comment: If many spermatozoa are attached to the zona pellucida of the oocytes, they can be removed by pipetting 20μL (using a 20μL pipette and a tip) 20-30 times in the fertilization dish before washing.
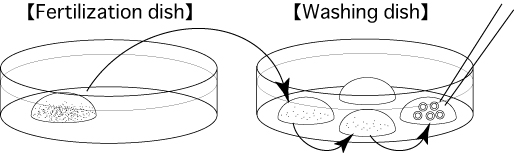
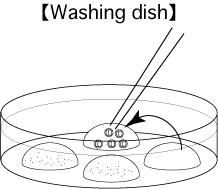
- 6 hours after insemination, observe them in the third drop of mHTF and remove any parthenogenetic oocytes which have only one pronucleus.
- After overnight culture of the oocytes, transfer the obtained 2-cell stage embryos only to the fourth drop of mHTF. These embryos can now be vitrified or transferred.
References
- Nakagata N., and Takeshima T. 1992. High fertilizing ability of mouse spermatozoa diluted slowly after cryopreservation. Theriogenol. 37: 1283-129.
- Nakagata N., Ueda S., Yamanouchi K., Okamoto K., Matsuda Y., Tsuchiya T., Nishimura M., Oda S., Koyasu K., Azuma S., and Toyoda Y. 1995. Cryopreservation of wild mouse spermatozoa. Theriogenol. 43: 635-643.
- Nakagata N. 1996. Use of cryopreservation techniques of embryos and spermatozoa for production of transgenic (Tg) mice and for maintenance of Tg mouse lines. Lab. Anim. Sci. 46: 236-238.
- Okamoto M., Nakagata N., Ueda O., Kamada N., and Suzuki H. 1998. Cryopreservation of gene disrupted mouse spermatozoa. J. Mamm. Ova. Res. 15: 77-80.
- Takeo T., Hoshii T., Kondo Y., Toyodome H., Arima H., Yamamura KI., Irie T., and Nakagata N. 2008. Methyl-beta-cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol Reprod. 78(3): 546-51.
- Takeo T., and Nakagata N. 2010. Combination medium of cryoprotective agents containing L-glutamine and methyl-β-cyclodextrin in a preincubation medium yields a high fertilization rate for cryopreserved C57BL/6J mouse sperm. Lab. Anim. 44(2): 132-7.
- Nakagata N. 2011. Cryopreservation of mouse spermatozoa and in vitro fertilization. Methods Mol Biol. 693: 57-73.
Update history
- Updated : 11 May, 2011
- Modified : 15 Jan, 2014
It is specified that incubation time after collection of oocytes is 30-60 minitues. For it, the procedures are rearranged. The procedures are Collection of oocytes -> thawing of sperm -> insemination. Old procedures are here. - Modified : 7 March, 2016
The recommended pipette tip for insemination was changed, because the old one was discontinued.
Old one: Cat.No.3520; Thermo SCIENTIFIC
New one: QSP pipette tip Cat.No.114 Thermo scientific


