In Vitro Fertilization (IVF) using Ultra-Superovulation Reagent ( From 2-cell to Blastocyst stage embryo )
Materials and Equipment
- Ultra-superovulation reagent (CARD HyperOva)
- hCG (human Chorionic Gonadotropin, CG-10; Sigma) (37.5IU/mL in sterile saline)
- 1mL disposable syringe
- FERTIUP® (Preincubation medium: PM; COSMO BIO)
- CARD MEDIUM (COSMO BIO)
- mHTF
- Micropipettes
- Pipette tips for preparation of dishes
- Pipette tips for insemination (Pipette Tip Cat.No.114; Quality Scientific Plastics)
- Plastic dishes (35mm x 10mm Cat.No.430588; CORNING)
- Fine scissors
- Pair of watchmaker's #5 forceps
- Micro-spring scissors (5mm blade)
- Dissecting needle
- Filter paper
- Glass capillaries for embryo handling
- Microscope
- Humidified incubator (37℃, 5% CO2, 95% air)
Procedure
Ultra-superovulation
- Induce superovulation by injecting 0.1-0.2mL of CARD HyperOva i.p. into a 26-30 days old female mouse (counting the date of birth as day 0).
(CARD HyperOva is usually administered during the light cycle, between the hours of 17:00 and 18:00). - Follow this up 48 hours later with a 7.5IU i.p. injection of human chorionic gonadotropin (hCG).
Preparation of Dishes
- Prepare dishes as per the instructions below and keep them in an incubator (37℃, 5% CO2 in air)
to allow them to gas-equilibrate.
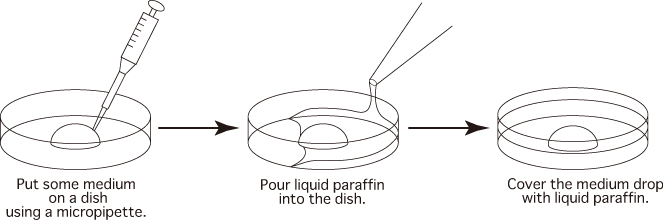
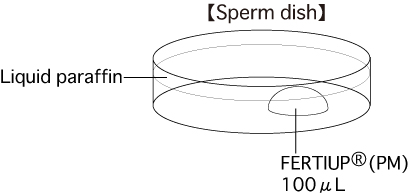
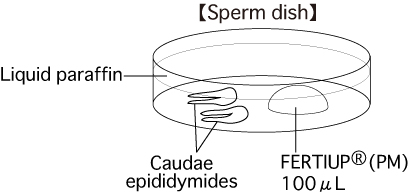
a. Sperm dishPut 1 drop (100μL / drop) of FERTIUP®(PM) into a dish and cover it with liquid paraffin 30 minutes before collecting sperm, and place the dish in an incubator.
b. Fertilization dishPut 1 drop (200μL / drop) of CARD MEDIUM into a dish and cover it with liquid paraffin 10 minutes before collecting oocytes, and place the dish in an incubator.
Note: There are three different methods of preparing CARD MEDIUM, depending on whether in vitro fertilization will be carried out using fresh, frozen-thawed or cold-temperature transported spermatozoa.
Please refer to the CARD MEDIUM instruction manual.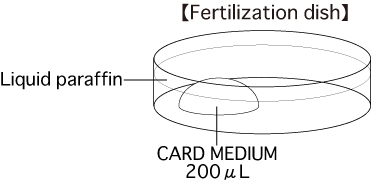
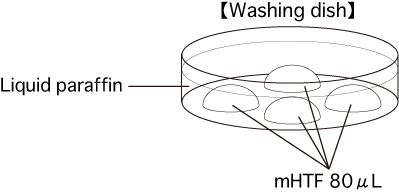
c. Washing dishPut 4 drops (80μL / drop) of mHTF into a dish and cover them with liquid paraffin. Place the dish in an incubator for at least 30 minutes.
Collection of Spermatozoa
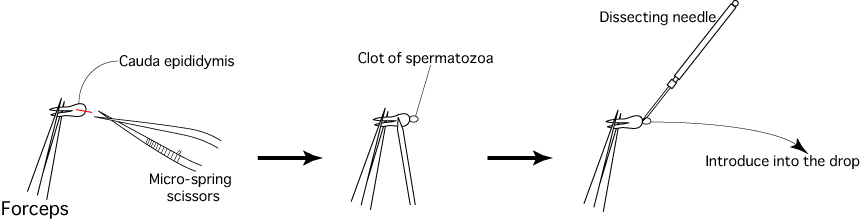
- Sacrifice 1 or 2 mature male mice (3 to 6 months old) and remove their cauda epididymides, avoiding as much fat, blood and tissue fluid as possible.
- Place the tissue on sterile filter paper to blot away any blood and fluid.
- Place the removed cauda epididymides in a sperm dish containing liquid paraffin.
- Cut the duct of each cauda epididymis using a pair of micro-spring scissors, then use a dissecting needle to gently press the surface of the cauda epididymis and release the sperm within.
- Use a dissecting needle to introduce the clots of spermatozoa released from the cauda epididymides into the drop of FERTIUP®(PM).
- Allow the sperm to capacitate by placing the suspension in an incubator (37℃, 5% CO2 in air) for 60 minutes before insemination.
| Note: | The degree of fertility varies greatly depending on the spermatozoa used. Spermatozoa with high fertility levels can be observed moving in a vortex with high motility at the boundary of the incubation medium. Conversely, spermatozoa which display low motility and poor homogeneity tend to have low fertility levels. |
Collection of Oocytes
- Sacrifice a superovulating female mouse approximately 15-17 hours after administering hCG.
- Dissect the mouse to expose the abdominal cavity.
- Move the digestive tract from inside the abdomen and expose the uteruses, oviducts and ovaries.
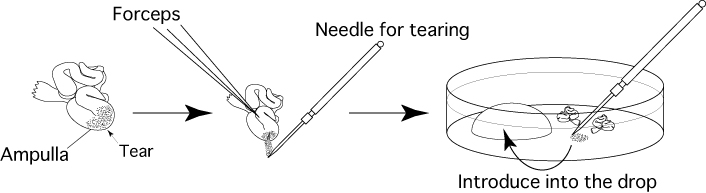
- Remove the oviducts (ampullae) only, and touch them on sterile filter paper lightly to remove blood and tissue fluid.
- Immerse the removed oviducts in liquid paraffin contained in a fertilization dish.
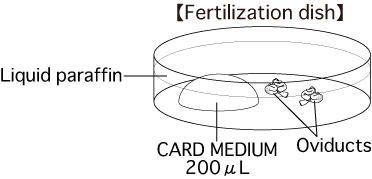
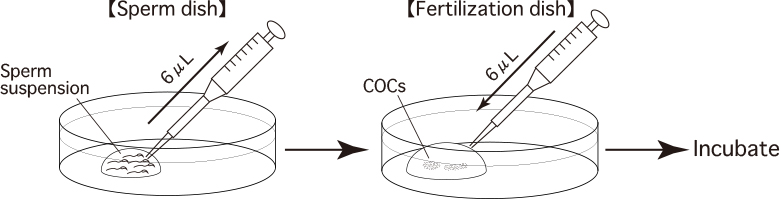
- Use forceps to hold the oviduct against the base of the fertilization dish, then use a dissecting needle to tear open the ampulla of the oviduct and release the cumulus-oocyte-complexes (COCs) from within. Drag them into the drop of CARD MEDIUM (200μL).
Note: Be sure to carry out all operations, from sacrificing the female and removing her oviducts to introducing the COCs into a drop of CARD MEDIUM, in the shortest time possible (within 30 seconds).
Moreover, when carrying out this process alone, do not sacrifice multiple mice at once; instead, sacrifice one mouse and swiftly remove its oviducts before moving on to the next mouse.
Use one drop of CARD MEDIUM (200μL) per female (2 oviducts).
- Keep the fertilization dish including COCs in an incubator (37℃, 5% CO2 in air) for 30-60 minutes before insemination.
Insemination
- Use the tip of a pipette (Pipette Tip Cat.No.114; Quality Scientific Plastics) to add appropriate amounts (usually about 6μL) of the sperm suspension to the drop of CARD MEDIUM containing the COCs.
- Place the fertilization dish in an incubator (37℃, 5% CO2 in air).
- 3 hours after insemination, wash the oocytes 3 times in fresh mHTF (80μL) in a washing dish, avoiding the transfer of CARD MEDIUM.
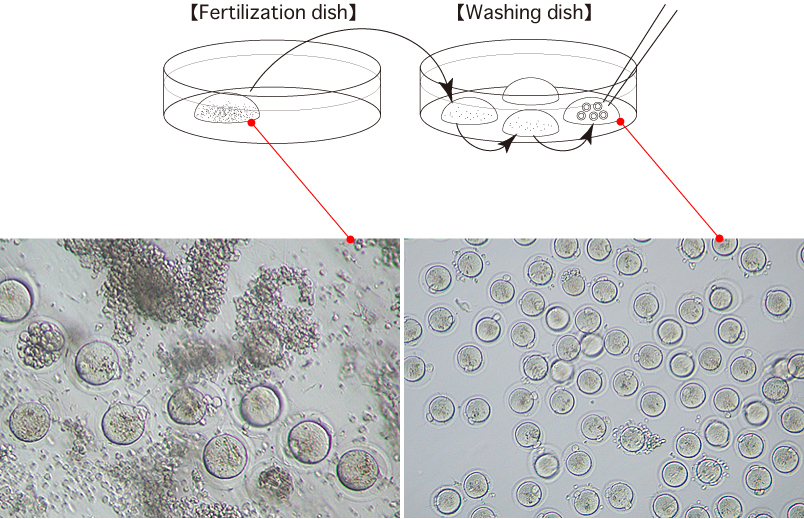
- 6 hours after insemination, observe the oocytes in the third drop of mHTF and remove any parthenogenetic oocytes which have only one pronucleus.
- After overnight culture of the oocytes, transfer the obtained 2-cell stage embryos only to the fourth drop of mHTF in a washing dish. These embryos can be vitrified, transferred to recipient females, or cultured to the blastocyst stage.
| Note: | At this stage it is important that you identify and remove any parthenogenetic oocytes. Please note that if you do not remove the parthenogenetic oocytes at this stage, the next day they develop to the 2-cell stage, at which point it will be impossible to distinguish the fertilized oocytes from the parthenogenetic oocytes. |
[Appearance of Fertilized, Unfertilized and Parthenogenetic Oocytes]
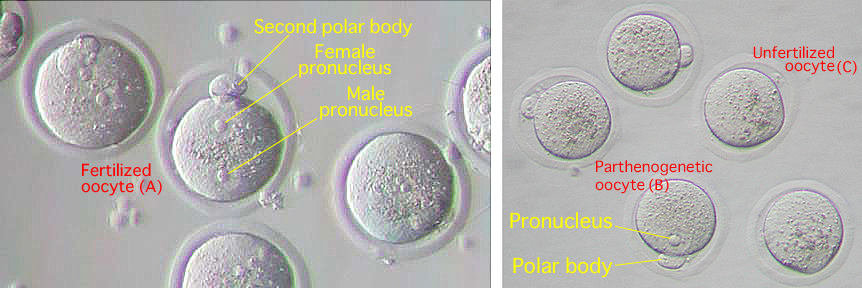

| Note: | The fertilized oocyte has both a male and female pronucleus (A). On the other hand, the parthenogenetic oocyte has only one pronucleus (B) and the unfertilized oocyte does not have any pronuclei (C). |
References
- Takeo T., Hoshii T., Kondo Y., Toyodome H., Arima H., Yamamura KI., Irie T., and Nakagata N. 2008. Methyl-beta-cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol Reprod. 78(3): 546-51.
- Takeo T., and Nakagata N. 2010. Combination medium of cryoprotective agents containing L-glutamine and methyl-β-cyclodextrin in a preincubation medium yields a high fertilization rate for cryopreserved C57BL/6J mouse sperm. Lab. Anim. 44(2): 132-7.
- Takeo T., and Nakagata N. 2011. Reduced glutathione enhances fertility of frozen/thawed C57BL/6 mouse sperm after exposure to methyl-beta-cyclodextrin. Biol Reprod. 85(5):1066-1072.
- Takeo T., Nakagata N. 2015 Superovulation Using the Combined Administration of Inhibin Antiserum and Equine Chorionic Gonadotropin Increases the Number of Ovulated Oocytes in C57BL/6 Female Mice.
PLoS One. 2015 May 29;10(5):e0128330. doi: 10.1371/journal.pone.0128330.
Update history
- Updated : 3 September, 2015
In case of superovulation reagent, the IVF protocol for the superovulation reagent differs from the protocol using PMS and hCG in two ways.
Firstly, the number of COCs to be placed in each fertilization dish ( include a drop of 200μL CARD MEDIUM ) shall be 2.
Secondly, the sperm suspention volume for insemination is about 6μL. - Modified : 16 March, 2016
In the old vide for collection of spermatozoa, not enough volume of soermatozoa was collected. That was not ideal, the new video was inserted. - Modified : 17 March, 2016
The experiments using CARD HyperOva injected into C57BL/6 demonstrated that 26-30 days old females ovulated more oocytes.
After it, the age for CARD HyperOva is changed from 25-29 days old to 26-30 days old. - Modified : 28 April, 2016
"Superovulation" is changed to "Ultra-superovulation".
Because CARD HyperOva can give more oocytes than PMSG, and to distinguish the new method from the traditional one. - Modified : 10 May, 2016
When using CARD HyperOva, the oviducts of superovulating female mice swell significantly.
Please be sure to handle the oviducts directly and carefully during "Collection of Oocytes" so as not to break them.
Old procedure:
- Remove the uteruses, oviducts and ovaries from the abdominal cavity of the female mouse.
- Place them on filter paper.
- Remove the oviducts (ampullae).
- Immerse them in liquid paraffin.
- Remove the oviducts (ampullae) from the abdominal cavity of the female mouse.
- Touch them on filter paper lightly to remove blood and tissue fluid.
- Immerse them in liquid paraffin.