Transport of 2-Cell Embryos at Cold Temperature
Materials and Equipment
- Two-cell embryos (adaptable for fresh and frozen/thawed embryos)
- Plastic dishes (35mm x 10mm Cat. No. 430588; CORNING)
- Gel-loading tip (Cat. No. 010-R204S; Bio Medical Instrument)
- M2 (Cat. No. M7167; Sigma)
- 0.5mL tube (Fisherbrand Flip Cap Microtubes 0.5mL; Fisher Scientific Cat. No. FS-MCT-060-C)
- Transfer pipettes
- KSOM/AA
- Temperature data logger (Thermochron iButton Cat.No.DS1921G; Maxim Integrated Products)
- CARD cold temperature transport kit (COSMO BIO)
- Thermos bottle (Cat.No.JMK-501; Thermos K.K.)
- Paper box (in which a 0.5mL tube can stand)
- Cotton wool
- Cold packs (small and large)
- Polystyrene foam transport box (KARUX KC-3)
Procedure
Cold Storage of 2-cell Embryos
- Place two 100μL drops of M2 on a plastic dish.
- Transfer the 2-cell embryos from the culture medium to the drop of M2.
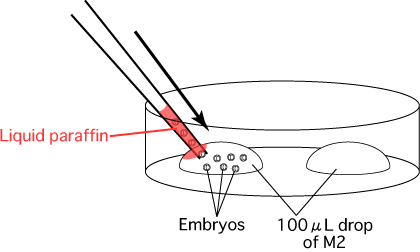
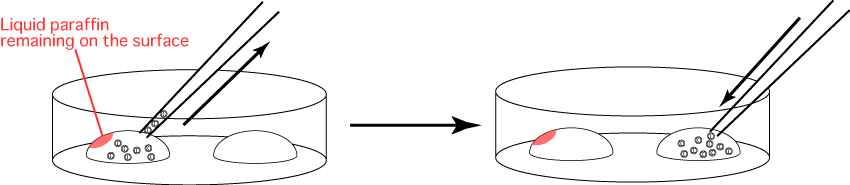
- Exchange the capillary for embryo manipulation and aspirate the embryos into the new capillary,
making sure to avoid dropping liquid paraffin onto the drop of M2.
Transfer the embryos into the drop of M2 made in step 1.
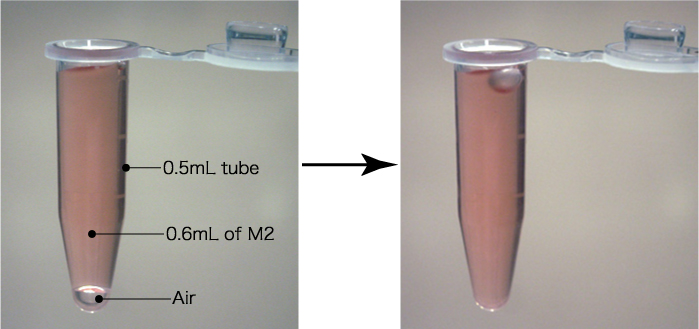
- Fill a 0.5mL tube with 0.6mL of M2 at room temperature. If there is a bubble at bottom of the tube, tap the tip of the tube and release the bubble.
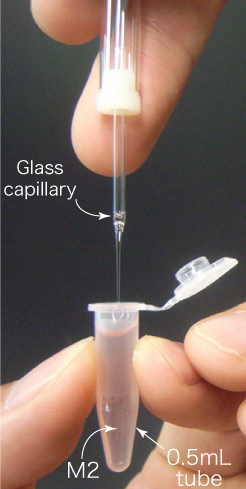
- Collect and transfer the embryos into the bottom of the tube (40 embryos/tube).
- Place the tube containing the embryos, a temperature data logger and a piece of cotton wool in the paper box.
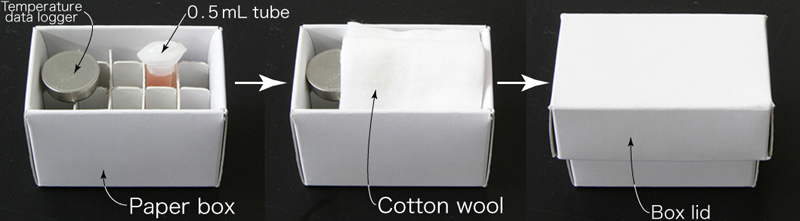
- Store the paper box in the refrigerator (4-8℃).
Comment: The embryos will maintain developmental ability for up to 72 hours.
Package and Transport of 2-cell Embryos
Prepare a paper box containing 2-cell embryos in the same manner as described above (Cold Storage of 2-cell Embryos).The cold packs (large) and a foam transport box must be precooled to 4-8℃ before use. Use the cold packs (small) and a thermos bottle at room temperature.
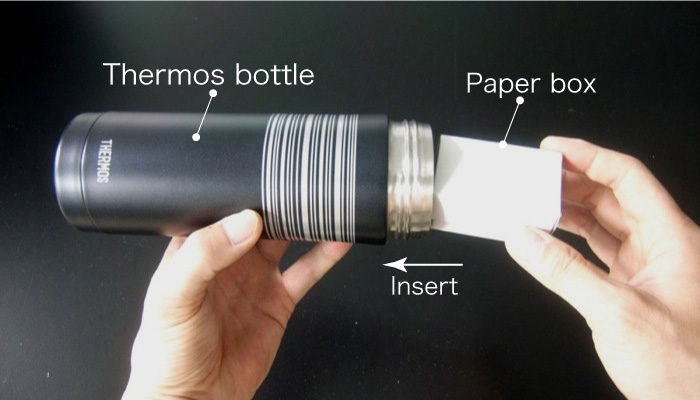
- Insert the paper box containing the embryos into a thermos bottle.
- Insert two cold packs (small) into the thermos bottle.
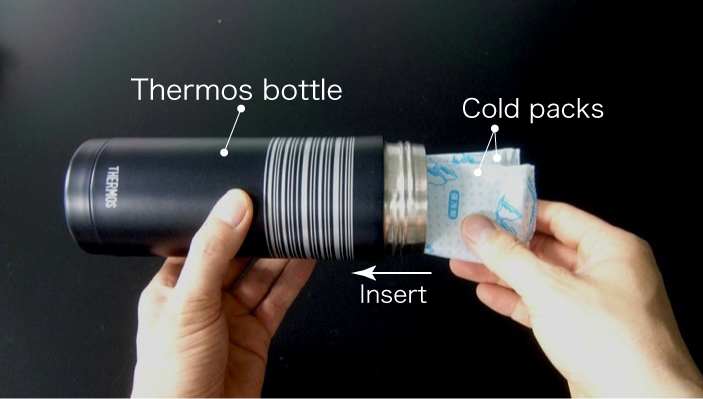
- Close the bottle cap.

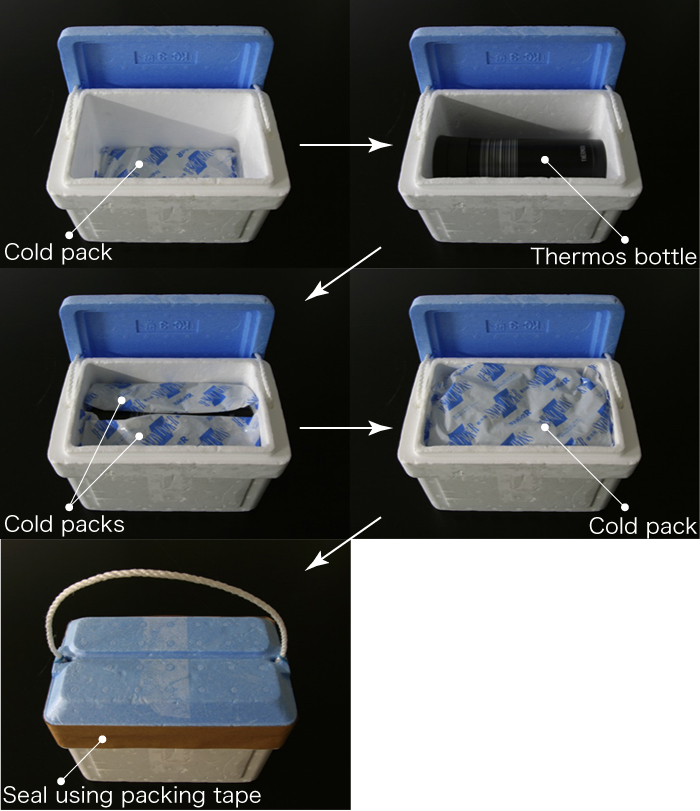
- Place a cold pack (large) at the bottom of a foam transport box, then put the thermos bottle on top of it.
- Pack one cold pack (large) on either side of the bottle, then set a further pack (large) on top and close the lid.
Note: Take care not to place the paper box upside down. Note: It is only possible to place the thermos bottle in the center of the foam transport box and not the actual bottom, because the length of the thermos bottle is the same as that of the inner length of the foam transport box. This is to protect the thermos bottle during shipping.
- Seal the lid of the foam transport box using packing tape.
- Keep the foam transport box in the refrigerator until a courier comes to pick it up.
- Send the samples via a regular courier service.
Note: The sample must be transferred at a refrigerated temperature. Please ask the courier service directly about conditions during transport. Comment: The embryos will maintain developmental ability for up to 72 hours.
Collection of 2-cell Embryos from the Transport Box
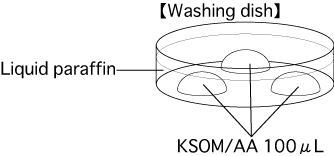
- Put 3 drops (100μL / drop) of KSOM/AA into a dish and cover them with liquid paraffin. Place the dish in an incubator (37℃, 5% CO2 in air) for at least 30 minutes.
- Retrieve the paper box containing the samples from the thermos bottle.
- Leave the paper box at room temperature for 30 minutes.
Comment: The embryos will sink to the bottom of the tube during those 30 minutes.
[Removing the sample]
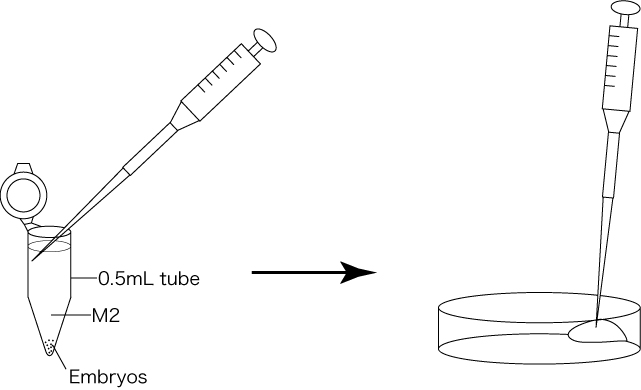
- Open the paper box and gently remove the cotton within. Once removed, pick up the tube containing the embryos and open it.
- Collect an upper layer of 200μL M2 from the tube using a gel-loading tip, then transfer the aliquot to the edge of the plastic dish.
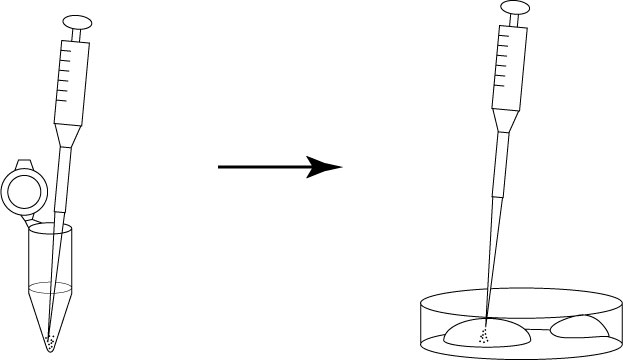
- Carefully retrieve all M2 containing the embryos from the bottom of the tube using a gel-loading tip, then transfer the aliquot to the center of the plastic dish.
Note: For easy manipulation, take care to avoid aspirating air bubbles in the gel-loading tip.
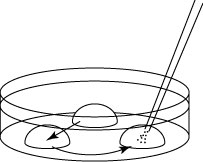
- Collect the embryos from the M2, then transfer and wash them in each of the three drops of 100μL KSOM/AA (washing dish).
Note: If you cannot retrieve all stored embryos, rinse the inside of the tube using the 200μL M2 at the edge of the plastic dish.
- Transfer the embryos into the oviducts of a pseudo-pregnant mouse.
Comment: Ideally, embryo transfer to pseudo-pregnant mice should be performed immediately upon the arrival of embryos.
References
- Takeo T., Kaneko T., Haruguchi Y., Fukumoto K., Machida H., Koga M., Nakagawa Y., Takeshita Y., Matsuguma T., Tsuchiyama S., Shimizu N., Hasegawa T., Goto M., Miyachi H., Anzai M., Nakatsukasa E., Nomaru K., and Nakagata N. 2009. Birth of mice from vitrified/warmed 2-cell embryos transported at a cold temperature. Cryobiology. 58(2): 196-202.
- Takeo T., Kondo T., Haruguchi Y., Fukumoto K., Nakagawa Y., Takeshita Y., Nakamuta Y., Tsuchiyama S., Shimizu N., Hasegawa T., Goto M., Miyachi H., Anzai M., Fujikawa R., Nomaru K., Kaneko T., Itagaki Y., and Nakagata N. 2010. Short-term storage and transport at cold temperatures of 2-cell mouse embryos produced by cryopreserved sperm. J Am Assoc Lab Anim Sci. 49(4): 415-419.
Update history
- Updated : 11 May, 2011
- Modified : 7 March, 2013
The order to put the paper box and cold packs into the bottle was changed. To avoid influence of external temperature, the paper box should be put in the back of the bottle.
Old procedure is here.


