In Vitro Fertilization (IVF) using Ultra-Superovulation Reagent ( Fertilized oocyte )
Materials and Equipment
- Ultra-superovulation reagent (CARD HyperOva)
- hCG (human Chorionic Gonadotropin, CG-10; Sigma) (37.5IU/mL in sterile saline)
- 1mL disposable syringe
- FERTIUP® (Preincubation medium: PM; COSMO BIO)
- CARD MEDIUM (COSMO BIO)
- mHTF
- Micropipettes
- Pipette tips for preparation of dishes
- Pipette tips for insemination (Pipette Tip Cat.No.114; Quality Scientific Plastics)
- Plastic dishes (35mm x 10mm Cat.No.430588; CORNING)
- Fine scissors
- Pair of watchmaker's #5 forceps
- Micro-spring scissors (5mm blade)
- Dissecting needle
- Filter paper
- Glass capillaries for embryo handling
- Microscope
- Humidified incubator (37℃, 5% CO2, 95% air)
Procedure
Ultra-superovulation
- Induce superovulation by injecting 0.2mL of CARD HyperOva i.p. into a 25-31 days old female mouse (counting the date of birth as day 0) or an adult female mouse (10-12 weeks old).
(CARD HyperOva is usually administered during the light cycle, between the hours of 17:00 and 18:00). - Follow this up 48 hours later with a 7.5IU i.p. injection of human chorionic gonadotropin (hCG).
| Note: | In the case of 25-31 days old mouse, body weight is important to induce ultra-superovulation. Ideal body weight is 14-18g. |
Preparation of Dishes
- Prepare dishes as per the instructions below and keep them in an incubator (37℃, 5% CO2 in air)
to allow them to gas-equilibrate.
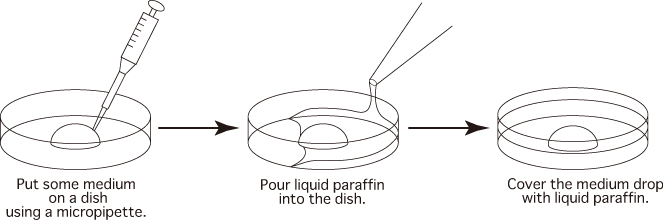
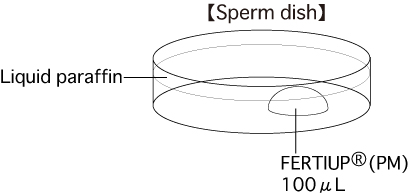
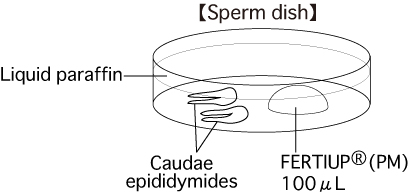
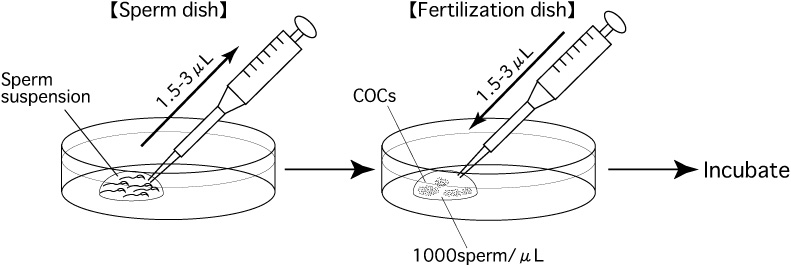
a. Sperm dishPut 1 drop (100μL / drop) of FERTIUP®(PM) into a dish and cover it with liquid paraffin 30 minutes before collecting sperm, and place the dish in an incubator.
b. Fertilization dishPut 1 drop (200μL / drop) of CARD MEDIUM into a dish and cover it with liquid paraffin 10 minutes before collecting oocytes, then place the dish in an incubator.
Note: To attain the appropriate effect on oocytes, prepare CARD MEDIUM to a low density.
Please refer to the CARD MEDIUM instruction manual for further details.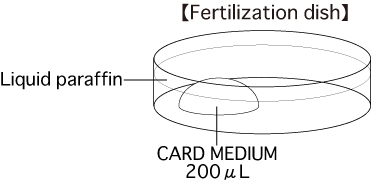
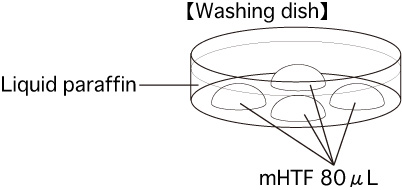
c. Washing dishPut 4 drops (80μL / drop) of mHTF into a dish and cover them with liquid paraffin. Place the dish in an incubator for at least 30 minutes.
Collection of Spermatozoa
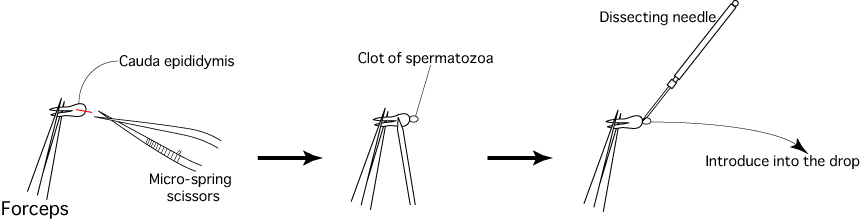
- Sacrifice 1 or 2 mature male mice (3 to 6 months old) and remove their caudae epididymides, avoiding as much fat, blood and tissue fluid as possible.
- Place the tissue on sterile filter paper to blot away any blood and fluid.
- Place the removed cauda epididymides in a sperm dish containing liquid paraffin.
- Cut the duct of each cauda epididymis using a pair of micro-spring scissors, then use a dissecting needle to gently press the surface of the cauda epididymis and release the sperm within.
- Use a dissecting needle to introduce the clots of spermatozoa released from the cauda epididymides into the drop of FERTIUP®(PM).
- Allow the sperm to capacitate by placing the suspension in an incubator (37℃, 5% CO2 in air) for 60 minutes before insemination.
| Note: | The degree of fertility varies greatly depending on the spermatozoa used. Spermatozoa with high fertility levels can be observed moving in a vortex with high motility at the boundary of the incubation medium. Conversely, spermatozoa which display low motility and poor homogeneity tend to have low fertility levels. |
Collection of Oocytes
- Sacrifice a superovulating female mouse approximately 15-17 hours after administering hCG.
- Dissect the mouse to expose the abdominal cavity.
- Move the digestive tract from inside the abdomen and expose the uteruses, oviducts and ovaries.
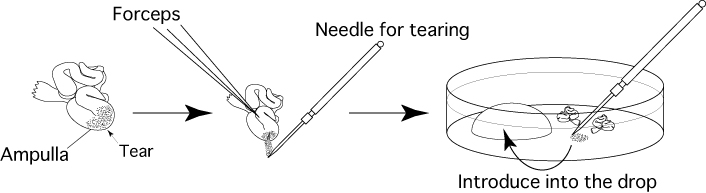
- Remove the oviducts (ampullae) only, and touch them on sterile filter paper lightly to remove blood and tissue fluid.
- Immerse the removed oviducts in liquid paraffin contained in a fertilization dish.
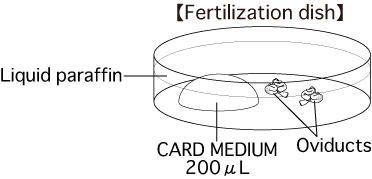
- Use forceps to hold the oviduct against the base of the fertilization dish, then use a dissecting needle to tear open the ampulla of the oviduct and release the cumulus-oocyte-complexes (COCs) from within. Drag them into the drop of CARD MEDIUM (200μL).
Note: Be sure to carry out all operations, from sacrificing the female and removing her oviducts to introducing the COCs into a drop of CARD MEDIUM, in the shortest time possible (within 30 seconds).
Moreover, when carrying out this process alone, do not sacrifice multiple mice at once; instead, sacrifice one mouse and swiftly remove its oviducts before moving on to the next mouse.
Use one drop of CARD MEDIUM (200μL) per female (2 oviducts) for 25-31 days old mouse.
Use one drop of CARD MEDIUM (200μL) per 2-3 females (4-6 oviducts) for adult mice (10-12 weeks old).
- Keep the fertilization dish including COCs in an incubator (37℃, 5% CO2 in air) for 20-40 minutes before insemination.
Insemination
- Among the prepared dishes of sperm, select the one which has the best motility and density of sperm.
- Use the tip of a pipette (Pipette Tip Cat.No.114; Quality Scientific Plastics) to add appropriate amounts
of the sperm suspension to the drop of CARD MEDIUM containing the COCs.
For example, if the expected number of oocytes in the fertilization dish is about 100, transfer 1.5-3μL of sperm suspension to obtain a final sperm density of about 1000/μL in the fertilization dish. - Place the fertilization dish in an incubator (37℃, 5% CO2 in air).
- 2.5 hours after insemination, wash the oocytes 3 times in fresh mHTF (80μL) in a washing dish, avoiding the transfer of CARD MEDIUM.
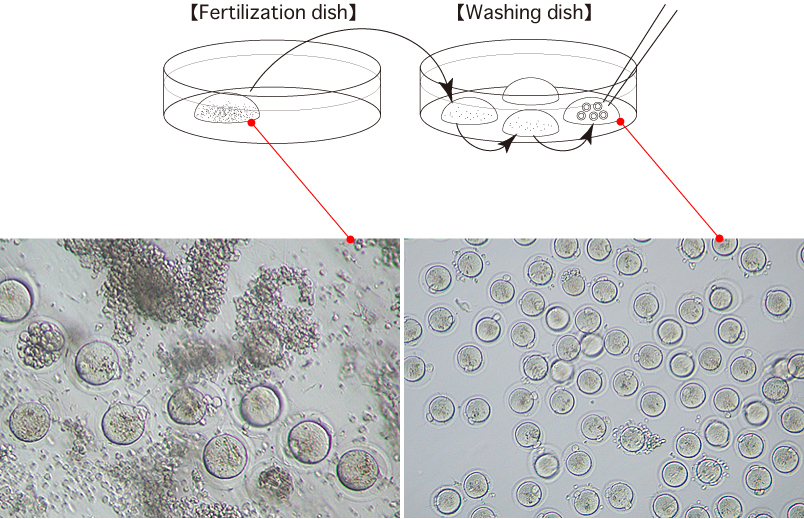
- 6-6.5 hours after insemination, observe the oocytes in the third drop of mHTF and transfer oocytes with a second polar body to a fourth drop of mHTF in a washing dish.
Please refer to the picture for an example of a second polar body in an oocyte. - The obtained oocytes can be vitrified following this procedure.
| Note: | It is difficult to select only fertilized oocytes in this step because their pronuclei cannot always be clearly observed. After warming the cryopreserved oocytes, it is possible to observe and select only fertilized oocytes. |
References
- Nakagawa Y., Sakuma T., Nakagata N., Yamasaki S., Takeda N., Ohmuraya M., Yamamoto T. 2014 Application of Oocyte Cryopreservation Technology in TALEN-Mediated Mouse Genome Editing. Exp. Anim. 63(3): 349-355.
- Nakagawa Y., Sakuma T., Sakamoto T., Ohmuraya M., Nakagata N., Yamamoto T. 2015 Production of knockout mice by DNA microinjection of various CRISPR/Cas9 vectors into freeze-thawed fertilized oocytes. BMC Biotechnol. 2015 15:33.
- Nakagawa Y., Sakuma T., Nishimichi N., Yokosaki Y., Yanaka N., Takeo T., Nakagata N., Yamamoto T. 2016 Ultra-superovulation for the CRISPR-Cas9-mediated production of gene-knockout, single-amino-acid-substituted, and floxed mice. Biol Open. 5(8): 1142-1148.
- Nakagawa Y., Sakuma T., Nishimichi N., Yokosaki Y., Takeo T., Nakagata N., Yamamoto T. 2017 Culture time of vitrified/warmed zygotes before microinjection affects the production efficiency of CRISPR-Cas9-mediated knock-in mice. Biol Open. 6(5): 706-713.
Update history
- Updated : 18 Aug, 2017
CARD MEDIUM is known to make the zona pellucida swollen and weak, so as to allow spermatozoa to easily penetrate into oocytes to fertilize them.
However, after IVF, the weakened zona pellucida cannot fully protect the oocyte from damage caused by manipulation for cryopreservation. Therefore it is important to correctly prepare oocytes using CARD MEDIUM.
Following the rules below will enable you to carry out in vitro fertilization and cryopreservation correctly.
- Prepare CARD MEDIUM following the method indicated in the instruction manual.
- Use an appropriate number of spermatozoa for fertilization. Refer to the 2nd step of the insemination procedure for further details.