Simple Vitrification of Mouse Oocytes
Materials and Equipment
- Female mice superovulated with PMSG and hCG
- Plastic dishes (35mm x 10mm Cat.No.430588; CORNING)
- Liquid paraffin
- Micropipette
- Pipette tips
- mHTF
- 1%Hyaluronidase in mHTF
- Fetal bovine serum(FBS Cat.No.26140-087; Gibco)
- Filter unit (Millex-GV 0.22μm Cat.No.SLGV013SL; MILLIPORE)
- Glass capillaries for embryo handling
- Humidified incubator (37℃, 5% CO2, 95% air)
- Materials and equipment used for vitrification and warming of embryos (For washing warmed oocytes, mHTF drops are used.)
Procedure
Preparation of Dishes
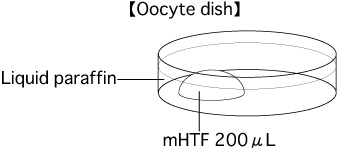
- Put a 200μL drop of mHTF into a dish. Cover it with liquid paraffin and place it in an incubator (37°C, 5% CO2 in air) for at least 30 minutes.
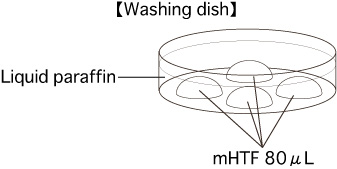
- Put 4 drops (80μL/drop) of mHTF into a dish. Cover them with liquid paraffin and place the dish in an incubator (37°C, 5% CO2 in air) for at least 30 minutes.
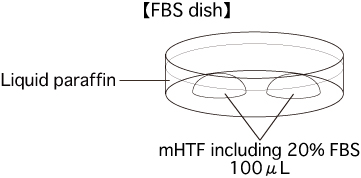
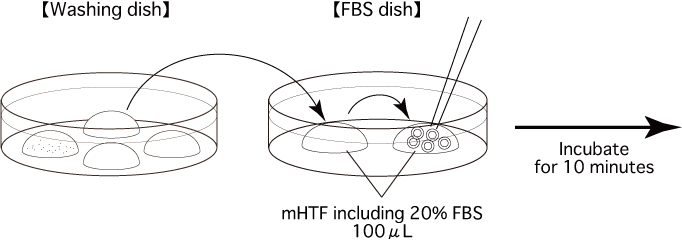
- Prepare some mHTF including 20% FBS, and sterilize it using a filter. Put 2 drops (100μL/drop) of the medium into a dish. Cover them with liquid paraffin and place the dish in an incubator (37°C, 5% CO2 in air) for at least 30 minutes.
Preparation of Denuded Oocytes
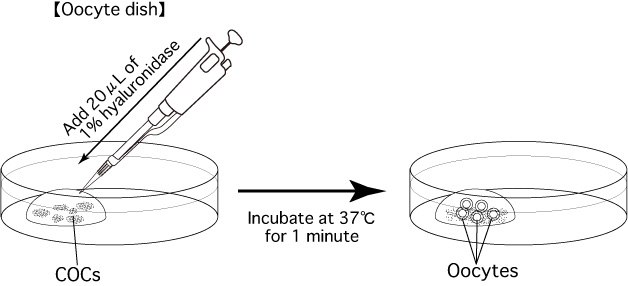
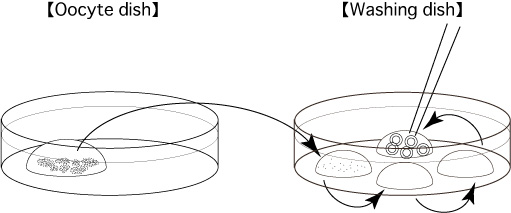
- Collect cumulus-oocyte complexes (COCs) from superovulated female mice and introduce them into a 200μL drop of mHTF (Oocyte dish).
Note: Be sure to carry out all operations, from sacrificing the female and removing her oviducts to introducing the COCs into a drop of mHTF (Oocyte dish), in the shortest time possible (within 30 seconds). Moreover, when carrying out this process alone, do not sacrifice multiple mice at once; instead, sacrifice one mouse and swiftly remove its oviducts before moving on to the next mouse. - Add 20μL of 1% hyaluronidase to the drop of mHTF containing the COCs, and keep the dish in an incubator (37°C, 5% CO2 in air) for 1 minute.
- Promptly collect and transfer the oocytes into a 80μL drop of mHTF (Washing dish), and in turn wash them in the drops in the washing dish.
Comment: If some cumulus cells become attached to the zona pellucida of the oocytes, they can be removed by manipulating the glass capillary.
Culturing Oocytes in a Drop Containing FBS
- Transfer the oocytes into the first drop in the FBS dish to rinse. Then, transfer them into the second drop to incubate (37°C, 5% CO2 in air) for 10 minutes.
Comment: FBS can prevent zona hardening in the oocyte during vitrifying and warming.
Simple Vitrification of Mouse Oocytes
- The oocytes can be vitrified using the simple vitrification method for embryos, after removing cumulus cells and culturing them in a drop containing FBS.
Moreover, the warming method is the same as for embryos.
Comment: mHTF drops are used for washing warmed oocytes.
In vitro Fertilization using Vitrified-Warmed Oocytes
- The vitrified-warmed oocytes can be used for in vitro fertilization using fresh, frozen-thawed and cold-temperature transported spermatozoa.
Note: There are three different methods of preparing CARD MEDIUM, depending on whether in vitro fertilization will be carried out using fresh, frozen-thawed or cold-temperature transported spermatozoa.
Please refer to the CARD MEDIUM instruction manual.
References
- Nakagata N, Takeo T, Fukumoto K, Kondo T, Haruguchi Y, Takeshita Y, Nakamuta Y, Matsunaga H, Tsuchiyama S, Ishizuka Y, Araki K. 2013. Applications of cryopreserved unfertilized mouse oocytes for in vitro fertilization. Cryobiology. Oct;67(2):188-92.
Update history
- Updated : 25 January, 2014


