Simple Vitrification of Fertilized Oocytes
Materials and Equipment
- Fertilized oocytes obtained from female mice which underwent ultra-superovulation
- 1M DMSO
- DAP213
- Plastic dish (35mm x 10mm Cat.No.430588; CORNING)
- Filter unit (Millex-GV 0.22μm Cat.No.SLGV013SL; MILLIPORE)
- Gel loading tip (MBP Gel 200, Cat.No.3621; Molecular BioProducts)
- Transfer pipettes
- Cryotubes (Cryogenic Vials Cat.No.MS-4501W; Sumitomo Bakelite, Japan is recommended. If you cannot get it, use 366656; NUNC.)
- Micropipette
- Vial canes
- Nalgene Labtop Cooler (5115-0012; NALGENE, USA)
- Liquid nitrogen
Procedure
Preparation of a Block Cooler and Cryotubes
- A day before use, place a block cooler in a freezer at -20℃.
- About 10 minutes before commencing the vitrifying procedure, take the block cooler out of the freezer.
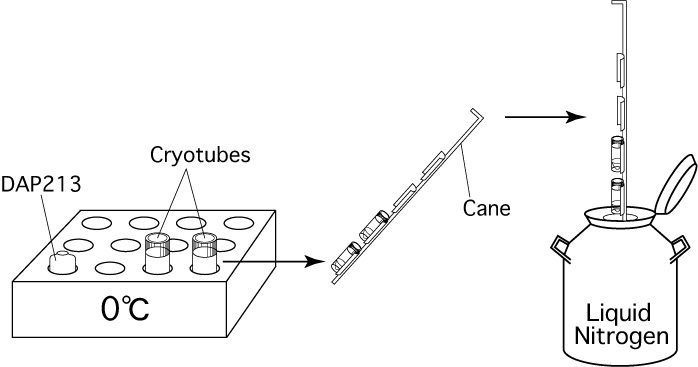
- Stand some cryotubes in the block cooler (5115-0012; NALGENE, USA).
- Just before starting the procedure, check that the temperature inside of the tubes is at 0℃.
Vitrification
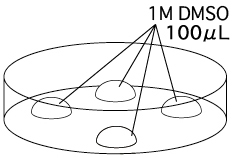
- Filter the 1M DMSO and put 4 drops of it (~100μL / drop) into a dish. Two drops
are to wash the oocytes taken from the collection medium, while the others are to hold the washed embryos.
- Place a group of 100 oocytes into one of the drops to rinse
them of the collection medium. Transfer the rinsed oocytes to the other
drops.
Once a drop is used to rinse or hold oocytes, do not use it again because 1M DMSO is diluted.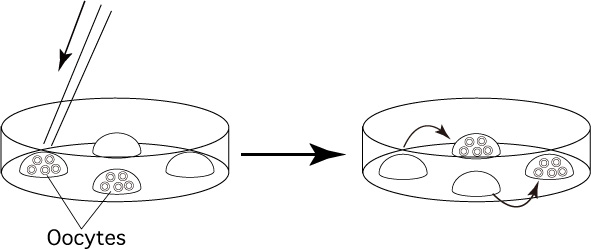
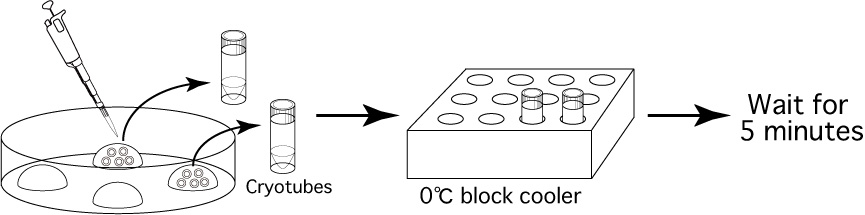
- Using a 20μL pipette and a gel-loading tip, transfer the
oocytes contained within 5μL of 1M DMSO solution
into a cryotube. Once transferred, put the cryotube into the block cooler at 0℃ and wait for 5 minutes.
Note: If the embryos are pushed together in the center of the drop, it is easy to suck them all up in 5μL of the 1M DMSO solution.
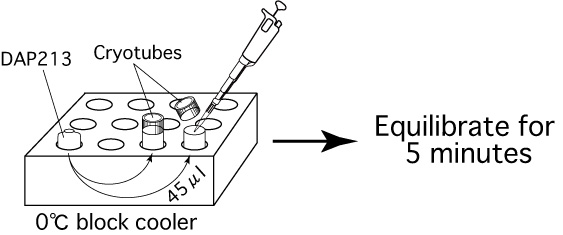
- Add 45μL of cryoprotective solution (DAP213)
at 0℃ into the cryotube and equilibrate for 5 minutes in the
0℃ block cooler.
Note: Do not fasten the caps too tightly after adding the DAP213, or they will be too difficult to remove quickly when samples are recovered from the freezer. - Quickly set the cryotubes on a cane and plunge the samples directly into liquid nitrogen.
Warming
- Recover the oocytes according to the procedure for 2-cell stage embryos.
References
- Nakagawa Y., Sakuma T., Nakagata N., Yamasaki S., Takeda N., Ohmuraya M., Yamamoto T. 2014 Application of Oocyte Cryopreservation Technology in TALEN-Mediated Mouse Genome Editing. Exp. Anim. 63(3): 349-355.
- Nakagawa Y., Sakuma T., Sakamoto T., Ohmuraya M., Nakagata N., Yamamoto T. 2015 Production of knockout mice by DNA microinjection of various CRISPR/Cas9 vectors into freeze-thawed fertilized oocytes. BMC Biotechnol. 2015 15:33.
- Nakagawa Y., Sakuma T., Nishimichi N., Yokosaki Y., Yanaka N., Takeo T., Nakagata N., Yamamoto T. 2016 Ultra-superovulation for the CRISPR-Cas9-mediated production of gene-knockout, single-amino-acid-substituted, and floxed mice. Biol Open. 5(8): 1142-1148.
- Nakagawa Y., Sakuma T., Nishimichi N., Yokosaki Y., Takeo T., Nakagata N., Yamamoto T. 2017 Culture time of vitrified/warmed zygotes before microinjection affects the production efficiency of CRISPR-Cas9-mediated knock-in mice. Biol Open. 6(5): 706-713.
Update history
- Updated : 18 Aug, 2017
For your convenience, handle the oocytes in groups of 100-130. The oocytes are obtained from females treated with CARD HyperOva, then equilibrated in 1M DMSO and cryopreserved in cryotubes.
In the equilibration dish containing 1M DMSO, use twice as many 1M DMSO drops as found in the cryotubes to equilibrate well.


