Simple Vitrification of Mouse Embryos
Materials and Equipment
- 1M DMSO
- DAP213
- Plastic dish (35mm x 10mm Cat.No.430588; CORNING)
- Filter unit (Millex-GV 0.22μm Cat.No.SLGV013SL; MILLIPORE)
- Gel loading tip (MBP Gel 200, Cat.No.3621; Molecular BioProducts)
- Transfer pipettes
- Cryotubes (Cryogenic Vials Cat.No.MS-4501W; Sumitomo Bakelite, Japan is recommended. If you cannot get it, use 366656; NUNC.)
- Micropipette
- Vial canes
- Nalgene Labtop Cooler (5115-0012; NALGENE, USA)
- Liquid nitrogen
Procedure
Preparation of a Block Cooler and Cryotubes
- A day before use, place a block cooler in a freezer at -20℃.
- About 10 minutes before commencing the vitrifying procedure, take the block cooler out of the freezer.
- Stand some cryotubes in the block cooler (5115-0012; NALGENE, USA). About 40 embryos / cryotube are easy to handle; in other words, when you want to vitrify 120 embryos, you need to stand 3 cryotubes in the block cooler.
- Just before starting the procedure, check the temperature inside of the tubes is at 0℃.
Vitrification
- Filter the 1M DMSO and put 4 drops of it (~100μL / drop) into a dish. One drop
is to wash the embryos taken from the collection medium, while the others are to hold the washed embryos.
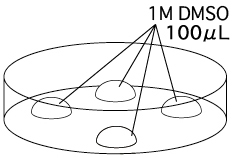
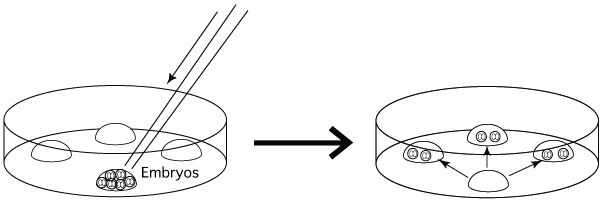
- Place a group of embryos into one of the 4 drops to rinse
them of the collection medium. Divide the rinsed embryos equally between the other
drops. These aliquots will eventually be transferred to a storage vial. For example, if one were to collect 120 embryos
and vitrify them in 40-embryo aliquots, the embryos would first be placed
together in the rinse drop and then divided equally among the three drops.
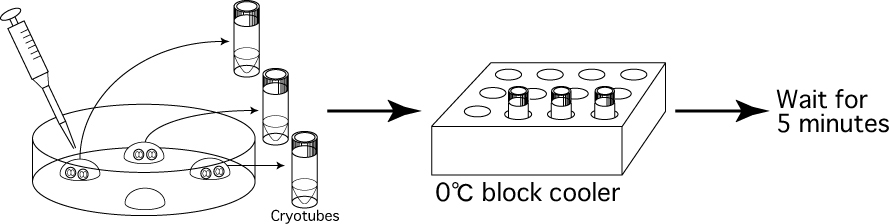
- Using a 20μL pipette and a gel-loading tip, transfer the
embryos contained within 5μL of 1M DMSO solution
into a cryotube. Once transferred, put the cryotube into the block cooler at 0℃ and wait for 5 minutes.
Note: It is possible to keep the cryotubes in the block cooler at 0℃ for longer than 5 minutes (<20 minutes). Note: If the embryos are pushed together in the center of the drop, it is easy to suck them all up in 5μL of the 1M DMSO solution.
- Add 45μL of cryoprotective solution (DAP213)
at 0℃ into the cryotube and equilibrate for 5 minutes in the
0℃ block cooler.
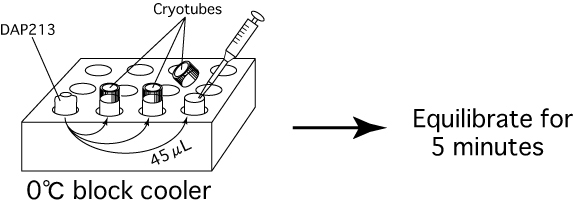
Note: Do not fasten the caps too tightly after adding the DAP213, or they will be too difficult to remove quickly when samples are recovered from the freezer. - Quickly set the cryotubes on a cane and plunge the samples directly into liquid nitrogen.
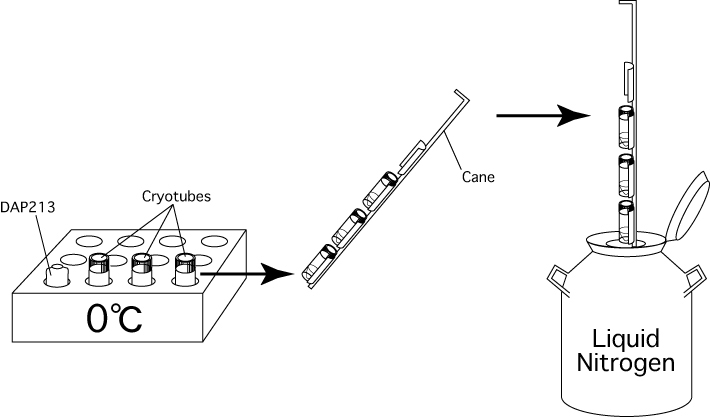
[Vitrifying Embryos]
References
- Nakao K., Nakagata N., and Katsuki M. 1997. Simple and effcient vitrification procedure for cryopreservation of mouse embryos. Exp. Anim. 46(3): 231-234.
Update history
- Updated : 11 May, 2011
- Modified : 16 January, 2013
In Materials & Equipment, recommended cryotube was changed. - Modified : 25 January, 2014
The procedures for oocytes has been moved to the other page.


